
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.6, Problem 11.14P
(a)
Interpretation Introduction
Interpretation:
The structures of other two possible products of linolenic acid is partially hydrogenated with one equivalent of hydrogen in the presence of a Pd catalyst has to be drawn.
Concept Introduction:
Hydrogenation reaction is nothing but the addition of hydrogen to an
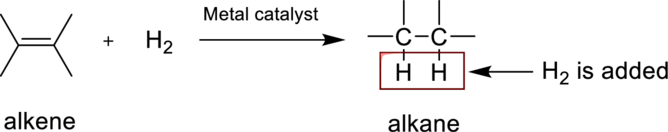
(b)
Interpretation Introduction
Interpretation:
The product formed when linolenic acid is completely hydrogenated with three equivalents of hydrogen has to be determined.
Concept Introduction:
Refer to part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write structural formulas for the products formed in each of the following reactions, and categorize the type of reaction involved
(a) CH3CH2CHO + (O) →
(b) CH3CH=CH2 + HBr →
Draw the structure for
a) (Z)-1-chloro-2-ethyl-1,3-butadiene
b) glycerol
c) (Z)-2-ethyl-2-buten-1-ol
Using condensed structural formulas, write a balancedchemical equation for each of the following reactions:(a) hydrogenation of cyclohexene, (b) addition of H2O totrans-2-pentene using H2SO4 as a catalyst (two products),(c) reaction of 2-chloropropane with benzene in the presenceof AlCl3.
Chapter 11 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 11.1 - Convert each condensed structure to a complete...Ch. 11.1 - Determine whether each molecular formula...Ch. 11.1 - Give the molecular formula for each of the...Ch. 11.2 - Give the IUPAC name for each alkene. a. (CH3CH2)2C...Ch. 11.2 - Prob. 11.5PCh. 11.2 - Give the structure corresponding to each name. a....Ch. 11.3 - Prob. 11.7PCh. 11.3 - Bombykol is secreted by the female silkworm moth...Ch. 11.3 - Prob. 11.9PCh. 11.3 - Prob. 11.10P
Ch. 11.3 - Prob. 11.11PCh. 11.5 - Prob. 11.12PCh. 11.5 - Prob. 11.13PCh. 11.6 - Prob. 11.14PCh. 11.6 - Prob. 11.15PCh. 11.7 - Prob. 11.16PCh. 11.7 - Prob. 11.17PCh. 11.9 - Prob. 11.18PCh. 11.9 - Draw the structure corresponding to each name. a....Ch. 11.10 - Prob. 11.20PCh. 11.10 - Prob. 11.21PCh. 11.10 - Prob. 11.22PCh. 11 - Prob. 11.23UKCCh. 11 - Prob. 11.24UKCCh. 11 - Prob. 11.25UKCCh. 11 - Prob. 11.26UKCCh. 11 - Answer the following questions about compound A,...Ch. 11 - Prob. 11.28UKCCh. 11 - Prob. 11.29UKCCh. 11 - Prob. 11.30UKCCh. 11 - Prob. 11.31UKCCh. 11 - Prob. 11.32UKCCh. 11 - Prob. 11.33APCh. 11 - Prob. 11.34APCh. 11 - Prob. 11.35APCh. 11 - Prob. 11.36APCh. 11 - Prob. 11.37APCh. 11 - Falcarinol is a natural pesticide found in carrots...Ch. 11 - Prob. 11.39APCh. 11 - Prob. 11.40APCh. 11 - Prob. 11.41APCh. 11 - Prob. 11.42APCh. 11 - Prob. 11.43APCh. 11 - Give the structure corresponding to each IUPAC...Ch. 11 - Leukotriene C4 is a key compound that causes the...Ch. 11 - Prob. 11.46APCh. 11 - Prob. 11.47APCh. 11 - Prob. 11.48APCh. 11 - Prob. 11.49APCh. 11 - Prob. 11.50APCh. 11 - Prob. 11.51APCh. 11 - Prob. 11.52APCh. 11 - Prob. 11.53APCh. 11 - Prob. 11.54APCh. 11 - Prob. 11.55APCh. 11 - Prob. 11.56APCh. 11 - Prob. 11.57APCh. 11 - Draw the products formed in each reaction.Ch. 11 - Prob. 11.59APCh. 11 - Prob. 11.60APCh. 11 - Prob. 11.61APCh. 11 - Prob. 11.62APCh. 11 - Prob. 11.63APCh. 11 - Prob. 11.64APCh. 11 - Prob. 11.65APCh. 11 - Prob. 11.66APCh. 11 - Prob. 11.67APCh. 11 - Prob. 11.68APCh. 11 - Prob. 11.69APCh. 11 - Prob. 11.70APCh. 11 - Prob. 11.71APCh. 11 - Prob. 11.72APCh. 11 - Prob. 11.73APCh. 11 - Prob. 11.74APCh. 11 - Prob. 11.75APCh. 11 - Prob. 11.76APCh. 11 - Prob. 11.77APCh. 11 - Prob. 11.78APCh. 11 - Prob. 11.79APCh. 11 - Prob. 11.80APCh. 11 - Prob. 11.81APCh. 11 - Prob. 11.82APCh. 11 - Prob. 11.83APCh. 11 - Prob. 11.84APCh. 11 - Prob. 11.85APCh. 11 - Prob. 11.86APCh. 11 - Are cis-2-hexene and trans-3-hexene constitutional...Ch. 11 - Prob. 11.88CP
Knowledge Booster
Similar questions
- Ethers (general formula R—O—R)have many important uses. Until recently,methyl tert-butyl ether (MTBE, right)was used as an octane booster and fueladditive for gasoline. It increases the oxy-gen content of the fuel, which reducesCO emissions. MTBE is synthesized bythe catalyzed reaction of 2-methylpropene with methanol.(a) Write a balanced equation for the synthesis of MTBE. (Hint:Alcohols add to alkenes similarly to the way water does.)(b) If the government required that auto fuel mixtures contain2.7% oxygen by mass to reduce CO emissions, how many gramsof MTBE would have to be added to each 100. g of gasoline?(c) How many liters of MTBE would be in each liter of fuel mix-ture? (The density of both gasoline and MTBE is 0.740 g/mL.)(d) How many liters of air (21% O₂ by volume) are needed at24C and 1.00 atm to fully combust 1.00 L of MTBE?arrow_forwardCharacterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true - false status of the statements using the choices. (1) Alcohols have higher boiling points than alkanes of similar molecular mass because of hydrogen bonding. (2) Polyols have similar boiling points to tertiary alcohols. (3) Primary and secondary alcohols give the same type of product when subjected to mild oxidizing agents. Group of answer choices All three statements are true. Two of the three statements are true. Only one of the statements is true. None of the statements are true.arrow_forward(a) Locate the isoprene units in lycopene, the red pigment in tomatoes. (b) Which isoprene units are connected in a head-to-tail fashion? (c) Label any other isoprene unit as connected in a head-tohead fashion or a tail-to-tail fashion. (d) Classify lycopene as a monoterpene, sesquiterpene, and so on.arrow_forward
- A)What is the IUPAC name for the product when hept-2-yne is reacted with two units of hydrogen gas over a platinum catalyst?B)In the complete combustion of octane the balanced reaction has one mole of the hydrocarbon. How many moles of oxygen are present?C)When 3,3-dimethylbutan-1-ol undergoes gentle oxidation, what is the product?arrow_forwardWrite chemical equations when (i) ethyl chloride is treated with alcoholic KOH. (ii) chlorobenzene is treated with CH3Cl in the presence of anhydrous AlCl3.arrow_forwardDraw structures corresponding to the following IUPAC names: (d) 4-Ethyl-2-propyloctanoic acid (e) 2-Cyclobutenecarbonitrilearrow_forward
- When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardDraw the structure and name the product formed if the following alcohols are oxidized. Assume an excess of the oxidizing agent is used. If the alcohol is not expected to react with a chemical oxidizing agent, write NR (no reaction).(a) CH3CH2CH2CH2OH(b) 2-butanol(c) 2-methyl-2-propanol(d) 2-methyl-1-propanolarrow_forwardKetene, H2C=C=O, is an important industrial chemical. Predict the products that would be formed when ketene reacts with **hint: Markovnikov addition occurs. (a) ethanol (b) acetic acid (c) ethylamine.arrow_forward
- What is the structure of the alcohol produced when 3-methyl-1-pentene undergoes (a) acid catalyzed hydration (b) oxymercuration/demercuration (c) hydroboration/oxidationarrow_forwardWhich products are formed when hydrobromic acid is added to (a) trans-2-hexene, (b) 2-methyl- 2-pentene, and (c) 4-methylcyclohexene, and how many regioisomers can be formed in each case?arrow_forward(a) Explain how bio-ethanol is produced?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning