
(a)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
(![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
The starting ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
Explanation of Solution
The given synthesis scheme is:
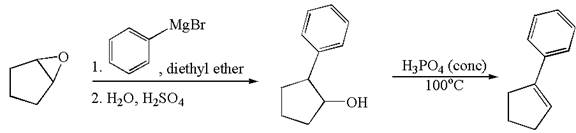
In the given synthetic route, the reactant having functional group epoxide is converted to an alcohol named ![]() which is further converted to an
which is further converted to an ![]() on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is
on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is ![]() which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and
which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and ![]() is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
The starting epoxide reacts with phenylmagnesium bromide in the solvent diethyl ether followed by aqueous acid, to form ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
The given synthesis scheme is converted to word form by identifying the names of reactants, reagents, and products.
(b)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to products by reacting with the reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane, to produce phenylpropanone. Then, add the molecular bromine in the presence of acetic acid to form ![]() which further reacts with sodium acetate to yield the final product.
which further reacts with sodium acetate to yield the final product.
Explanation of Solution
The given synthesis scheme is:
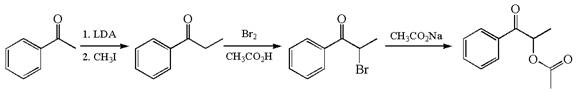
The given synthetic route is of three steps. In the first step, the reactant having functional group ![]() alpha bromo ketone named
alpha bromo ketone named ![]() , which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
, which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane to produce phenylpropanone. Add the molecular bromine in presence of acetic acid to phenylpropanone to form ![]() which further reacts with sodium acetate to yield THE final product.
which further reacts with sodium acetate to yield THE final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
(c)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , add phosphorous tribromide to it to produce
, add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
Explanation of Solution
The given synthesis scheme is:
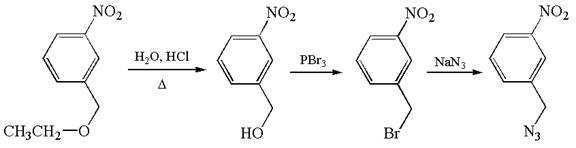
The given synthetic route is of three steps. The nitro functional group remains as it is throughout the reaction sequence, and so, is not considered. In the first step, ether functional group is converted to an alcohol. In the second step, ![]() group of alcohol is replaced by bromine, which in the third step is replaced by
group of alcohol is replaced by bromine, which in the third step is replaced by ![]() . In the first step, the reagent used is
. In the first step, the reagent used is ![]() that represents acidic condition where
that represents acidic condition where ![]() is the symbol used for heat. In the second step, the reagent is
is the symbol used for heat. In the second step, the reagent is ![]() , phosphorous tribromide, and in the third step, the reagent used is sodium azide,
, phosphorous tribromide, and in the third step, the reagent used is sodium azide, ![]() . Thus, the word form of the above synthetic scheme can be written as follows:
. Thus, the word form of the above synthetic scheme can be written as follows:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , and add phosphorous tribromide to it to produce
, and add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Show how to bring about each step in this synthesis of the herbicide propranil.arrow_forward2. Prepare the following compound start from 1-methylenecyclopentane, 1-pentyne, and any other reagents you may need.arrow_forwardEach of the following is a set of directions for carrying out a reaction or sequence of reactions. Rewrite each set ofdirections in the form of a synthesis.(a) To 2-ethylcyclohexanone, add lithium diisopropylamide, and when that reaction is complete, add bromoethane toyield 2,6-diethylcyclohexanone.(b) Add molecular bromine to 2,2-dimethylcyclohexanone in the presence of acetic acid to yield 6-bromo-2,2-dimethylcyclohexanone. To the resulting mixture, add sodium cyanide to yield 6-cyano-2,2-dimethyl-cyclohexanone.(c) Treat pent-4-ynoic acid with diazomethane to produce methyl pent-4-ynoate. Next, add sodium hydride, followed by(bromomethyl)benzene, to yield methyl 6-phenylhex-4-ynoate.arrow_forward
- Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. H3C NH₂ HCI/H₂O reflux • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Na+, I, in your answer. • In cases where there is more than one answer, just draw one.arrow_forwardA chemist needs an ether to use as a solvent for a reaction and wants to synthesize it in one step from two of the following available reagents: sodium ethoxide, bromomethane, potassium tert-butoxide, and 2-bromo-2-methylpropane. i) Which combination(s) will give a good yield of an ether? Illustrate, showing the mechanism of the reaction. ii) Illustrate with a mechanism the reaction of one of the combinations that will not yield an ether?arrow_forwardShow a possible stepwise synthesis of 2, 3-dichloropentane starting with ethanol and propanol as the only sources of carbon atoms and any other inorganic reagents, solvents and laboratory equipment. Name the type of reaction that is occurring in each step of the synthesis.arrow_forward
- Convert 2-Phenylethyl bromide to 2-Bromo-1-phenylethanol using any necessary organic and inorganic reagents. It will require multiple steps.arrow_forwardThe synthesis above used bromoethane, but acetylene is the only allowed source of carbon atoms. Using the reagents given, identify a synthetic route for the production of bromoethane from acetylene. H- Br The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as “EBF"). If there is more than one correct solution, provide just one answer. A В МeONa NaNH2 Н2, Pt D E F HBr H2, Lindlar's cat. Melarrow_forwardAcyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. You do not have to consider stereochemistry. Include all valence lone pairs in your answer. Do not include counter-ions, e.g., Na+, I-, in your answer. In cases where there is more than one answer, just draw one.arrow_forward
- 2. Provide the reagents required to complete the following multi-step synthesis. Write the structure of the product after each synthetic step.arrow_forwardProvide the reagents for the short syntheses (about 3-4 steps).arrow_forwardComplete the synthesis of ethyl (E)-2,2,4-trimethyl-3-oxo-5-phenylpent-4-enoate from the starting material given (ethyl propionate). You must list out all reagents/solvents next to the reaction arrows and draw the intermediate structures in the boxes. I am giving you one restriction. You are not allowed to introduce any enolates as reagents next to the reaction arrows.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
