
a)
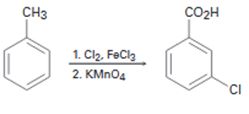
Interpretation:
The flaw in the synthesis given is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, alkyl groups are ortho and para directing and activating groups while
To state:
The flaw in the synthesis given.
b)
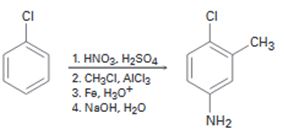
Interpretation:
The flaw in the synthesis given is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, halogens are ortho and para directing groups. Friedal-Crafts alkylation is not possible with
To state:
The flaw in the synthesis given.
c)
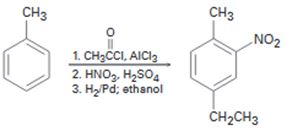
Interpretation:
The flaw in the synthesis given is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, alkyl groups are ortho and para directing and activating groups while carbonyl group is a meta directing and deactivating group. When treated with H2/Pd the –C=O group gets reduced to –CH2 group and –NO2 gets reduced to –NH2 group.
To state:
The flaw in the synthesis shown.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- 1) a until garrow_forwardChemistry 3. Provide reagents/conditions to accomplish the following syntheses. Several steps are required in some cases. (c) (d) H2N. NH2 но H. NH2 ноarrow_forwardPropose the starting material Wμ H30+, heat a) d) c) NH₂ P Suggest reagents EN якое a) CH 3 CH ₂ MgBr, 2. H3O+ b)CH3CH2, NaH, 2. H₂Oz C) BH3, 2. H₂O2 | NaOH α) H30+, D HOarrow_forward
- The following synthetic schemes all have at least one flaw in them. What is wrong with each? CH, „COOH 1. BH, 2. H,0 (a) ÇOOH 1. Mg 2 NaCN 3. H,0* (b) CH,CH,CHBrCH¿CH, CH,CH,CHCH,CH, „CH2COOH „CH,CH 1. LIAIH, 2. H,0* (e)arrow_forwardFor question 2a and 2b below, which of the puzzle pieces makes the best sequence for each synthesis? You must solve the syntheses with the puzzle pieces provide and nothing else. One piece can only be used once.arrow_forwardwhat predictions can we make of the following reactionsarrow_forward
- (b) Predict the suitable solvent (H2O or CH3COCH3) to increase the reaction of bromopropane (CH3CH2CH2B1) with sodium hydroxide (NaOH). Two reactions are shown below: NaOH, 55 °C CH;CH,CH,Br CH;CH,CH,OH + NaBr H,O (i) NaOH, 55 °C CH;CH,CH,Br CH;CH,CH,OH NaBr H,C CH (ii)arrow_forward1. How could you fix the reaction below so that it gives the desired product? ( OH 1. Mg, EtO CH3 2. HC, Н,О Not Observedarrow_forwardDoes the reaction in part b have multiple possible products? Do you know which one is more abundant?arrow_forward
- 7. The reaction of methoxide anion with bromoethane to yield the ether ethyl methyl ether and the bromide anon (Br-) is an excellent example of a general reaction type called Sy2 (substitution nucleophilic bimolecular): CH,0+ CH,СH-Br a CH3-0-CH,СH; + Br- a. Change in enthalpy is -103 kJ/mol; the change in entropy is + 0.025 kJ/mol-K. Calculate DG at 300K. b. Is the reaction endergonic or exergonic? c. Is the reaction endothermic or exothermic? d. Use curved arrows to show the complete mechanism. Reaction of 2-methyl-1-butene with H-Cl could yield TWO alkyl chloride products. Draw and name 8. them.arrow_forwardWorksheet 1: Structural Formulas and Reactions in Organic Chemistry Provide the necessary information for the table below Condensed Structure (CH3)2CHCH2CH2CH(CH3)2 CH3CH(CI)CH(OH)CH3 CH3(CH2)2C(CH3)2CH(CH3)CH(CH3)CH(Br)CH3 CH3COCI CH3COCH₂Br Dash line Structure Bond line Structurearrow_forwardFollowing is the structural formula of the tranquilizer meparfynol (Oblivon). Propose a synthesis for this compound starting with acetylene and a ketone. (Notice the -yn- and -ol in the chemical name of this compound, indicating that it contains alkyne and hydroxyl functional groups.)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
